148
Views & Citations10
Likes & Shares
According to the site of involvement, tuberculosis is classified as pulmonary and extrapulmonary tuberculosis (EPTB). Approximately 20 to 25 percent of all TB cases are due to EPTB. Lymph nodes, followed by TPE, are the most common sites of involvement in EPTB patients [2]. TPE contributes to 30% to 80% of all pleural effusions. TPE usually has lymphocytes predominant and exudative. Exudation occurs due to pleural inflammation followed by increased permeability to protein-containing fluid and different kinds of cells in the pleural space. Sometimes lymphatic obstruction may contribute to pleural fluid accumulation [3].
According to Light’s criteria, 99% of pleural effusion may be categorized into exudative and transudative [4]. In comparison with transudative effusion, the cause and management of exudative effusion are difficult. Due to the low sensitivity of several conventional procedures, diagnosing TPE is difficult. The presence of tuberculous basil in sputum, pleural fluid, a sample of pleural tissue, or a histological finding of a caseating granuloma in pleural tissue might confirm the diagnosis [5]. Pleural fluid AFB demonstration is virtually negative, positive only 5%; Culture positivity is present in only 15% to 20% of pleural fluid and nearly 60% to 80% of pleural biopsy samples [6]. Pleural biopsy is an invasive technique and is not available everywhere. Different types of biomarkers are used for the diagnosis of TPE among them adenosine deaminase (ADA) in pleural effusion is the most useful for early diagnosis. There are several isoforms of ADA, in TPE, ADA2 isoforms are predominant, accounting for most of the total ADA [7]. A raised ADA level has a 90% sensitivity and a 50% specificity for predicting TPE [8]. However, ADA is not only high in TPE, but an elevated value is also found in other infections, lymphoma, connective tissue disease, and malignancy [9]. Polymerase chain reaction (PCR) analyses for the amplification and recognition of MTB nucleic acids in pleural effusion have low sensitivity (62%) but high specificity (98%) [10].
Interferon-gamma (IFϒ) is a cytokine produced by stimulated CD4+ T lymphocytes and enhances macrophages' mycobactericidal activity. Interferon-gamma Release Assays (IGRAs) in TPE had a sensitivity and specificity of 75% and 82%, respectively. IGRAs are expensive, technically challenging tests that frequently yield undesirable false-positive and false-negative results [11]. Mycobacterial protein and glycolipid antibodies can be used in serological assays to diagnose TPE, because of their high specificity nonetheless are limited by low sensitivity [12]. Different researchers have studied the evaluation of cholesterol in the pleural fluid. However, it is unknown what has caused the cholesterol levels in pleural fluid to increase. There had been put forth two potential theories. Firstly, pleural fluid cholesterol is increased due to cellular degeneration mainly of white and red blood cells. Secondly, cholesterol is derived from serum because of the increased permeability of pleural capillaries in exudative pleural effusions [13].
Pleural fluid cholesterol is used to categorize exudative and transudative pleural effusion as it misclassifies less frequently than any other Light’s parameters. Different studies showed that pleural fluid cholesterol was significantly associated with tuberculous pleural effusion [14-16]. However, Studies evaluating the diagnostic potential of pleural fluid cholesterol in TPE are scarce Therefore, this study was designed to explore the potential diagnostic value of pleural fluid cholesterol for TPE.
MATERIALS AND METHODS
Study Design and Population
This cross-sectional comparative study was carried out at the Department of Respiratory Medicine at BSMMU from February 2022 to January 2023. A total of seventy participants diagnosed with exudative pleural effusion aged >18 years were included. Pleural biopsy was done to confirm TPE. Patients were excluded if they had nephrotic syndrome, chylothorax, lymphoma, blood diathesis, malnourishment, and who were on lipid-lowering agents.
Ethical Approval
The Institutional Review Board (IRB) of BSMMU, Bangladesh, approved the study proposal (BSMMU/2022/1279/3740). The study's protocol adhered to the Helsinki Declaration's ethics guidelines. Before the enrolment, written informed consent was obtained from all the participants.
Specimen Collection and Measurement
After obtaining informed written consent, a thorough history and physical examination were done. Pleural fluid aspiration followed by pleural biopsy was done by using Abraham’s needle at sitting forward, leaning on a pillow over the table, with their arms folded in front of them. In a sterile test tube, pleural fluid was collected and sent for the cytological, microbiological, and biochemical study, which includes total protein, lactate dehydrogenase (LDH), glucose, and ADA. On the other side, pleural tissue was collected in formalin for histopathological examination. At the same time, 5ml of blood was collected from the antecubital vein and sent for complete blood count, serum protein, glucose, and LDH. Pleural fluid cholesterol was measured by an automated analyzer (Architect Plus ci4100) in the Department of Biochemistry of BSMMU.
Other Data Collection
A structured data collection sheet was used to collect the data. Demographic (age, and gender), comorbidity (Hypertension, diabetes, chronic liver disease, chronic kidney disease, rheumatoid arthritis, and smoking), and clinical features such as fever, cough, chest pain, breathlessness, hemoptysis, and weight loss were documented.
Statistical Analysis
After data collection, all data were reviewed, tallied, and coded. The data were compiled and statistical analysis was carried out using the SPSS-23 version of the software [17]. The categorical variables (Gender, etiology of non-TPE, presenting complaints, co-morbidity, and risk behavior) were described as frequency and percentages. Chi-squared test or Fisher's exact test was used to compare the group differences. Continuous variables (Age, laboratory, pleural fluid parameters including pleural fluid cholesterol) as mean ± SD or median. To compare the mean variations between the two groups student’s t-test or Mann-Whitney U test was used. Receiver operator characteristic (ROC) curve analysis was used to assess the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of pleural fluid cholesterol to diagnose TPE. The risk factor for elevated pleural fluid cholesterol was determined using binary logistic regression. A value of p<0.05 was considered statistically significant.
RESULTS
Patient Characteristics
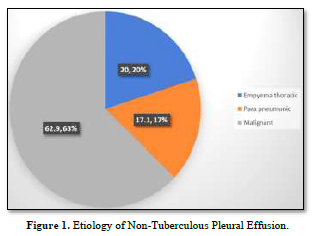
This study comprised a total of 70 patients with exudative pleural effusion, of which 35 had biopsy-proven TPE and 35 non-TPE. The mean age of TPE was significantly lower than non-TPE and was male-predominant (71.4%). The most frequent presenting symptoms in non-TPE participants were cough, breathlessness, and chest pain; fever and weight loss were more common in participants with TPE. Between the two groups, there was no significant difference in the distribution of comorbidity. The demographic, presenting complaints and co-morbidity of the participants are presented in Table 1.
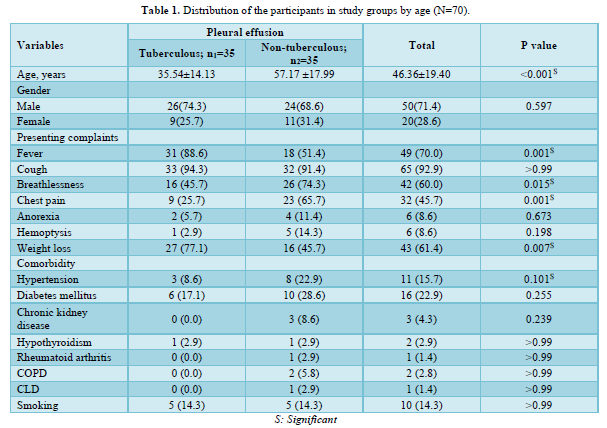
Etiology of non-tuberculous pleural effusion
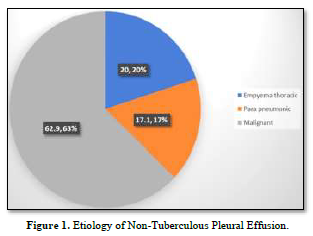
Figure 1 demonstrates the etiology of non-TPE. Among the participants 7 out of 35 (20.0%) were suffering from empyema thoracic, 6 out of 35 (17.1%) were from para-pneumonic effusion and 62.9% were suffering from malignant pleural effusion.
General and Systemic Examination Findings of the Participants
Almost one-third of the participants were suffering from anemia (31.4%), whereas the proportion of anemia was significantly higher among participants suffering from non-TPE (45.7%). There was no discernible difference between the groups in terms of clubbing, jaundice, pulse, systolic blood pressure, diastolic blood pressure, or respiratory rate (p-value >0.05).
Laboratory Findings of the Study Subjects
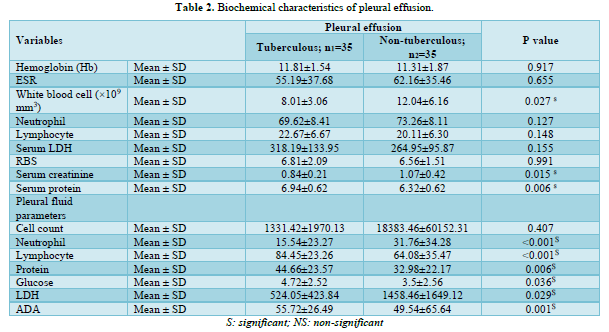
White blood cell count (×109 mm3) and serum creatinine were observed to be considerably higher in the non-TPE group than in the TPE groups (p-value: <0.05). Contrary, serum protein was found significantly higher among the TPE than in non-TPE (p-value: 0.015). Pleural fluid lymphocyte count, protein, glucose, and ADA were notably greater in the TPE group, whereas neutrophil count and LDH level were higher in the non-TPE group (p-value <0.05). Biochemical characteristics are demonstrated in Table 2.
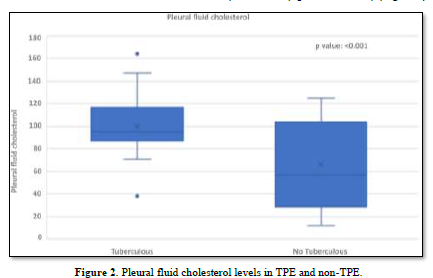
Pleural Fluid Cholesterol of TPE and Non-TPE
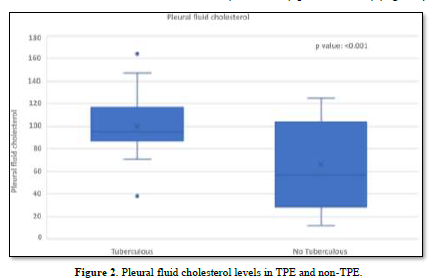
The concentration of pleural fluid cholesterol in TPE (99.87±23.82) was significantly higher than in non-TPE (66.33±36.89) (p-value < 0.001) (Figure 2).
ROC Analysis of Pleural Fluid Cholesterol and ADA
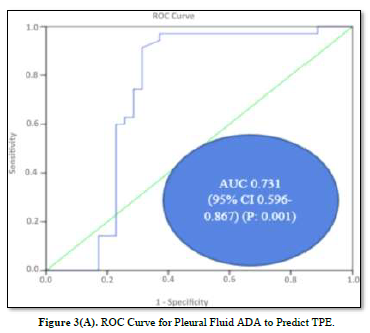
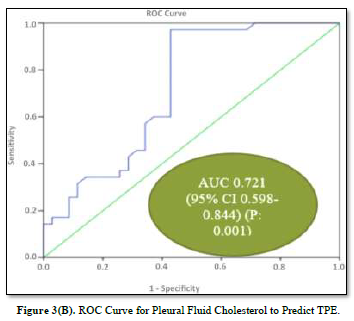
ROC curves for the analysis of pleural fluid ADA and cholesterol to predict TPE are plotted in Figures 3(A) & 3(B). The AUC of pleural fluid cholesterol (0.721, 95% CI: 0.598-0.844) was identical to the AUC of pleural fluid ADA (0.731, 95% CI: 0.596-0.867), which was statistically significant (P value: 0.001).
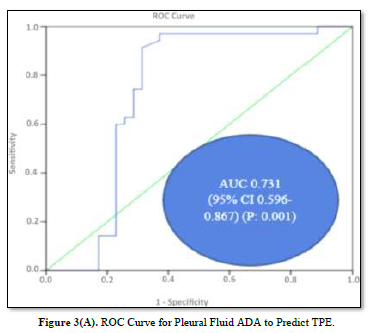
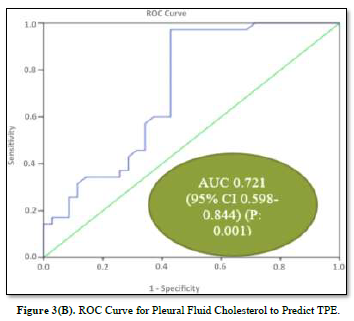
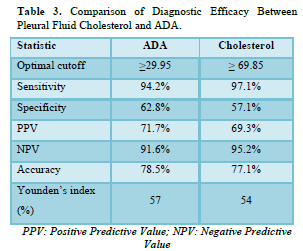
Diagnostic Potential of Pleural Fluid Cholesterol for TPE
The diagnostic values of pleural fluid ADA and cholesterol are displayed in Table 3. ADA demonstrated the highest Youden index 0.57 with a cut-off value of 29.95 IU/L pleural fluid, 94.2% sensitivity, 62.8% specificity, 71.7% PPV, 91.6% NPV, and 78.5% accuracy. On the other hand, pleural fluid cholesterol displayed the highest Youden index 0.54 with a cut-off value of 69.85, exhibiting 97.1% sensitivity, 57.1% specificity, 69.3% PPV, 95.2% NPV, and 77.1% accuracy.
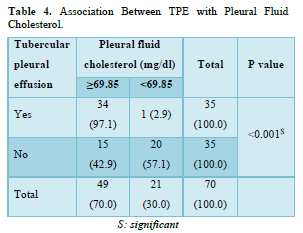
Association Between TPE and Pleural Fluid Cholesterol
In our investigation, a strong association between TPE and pleural fluid cholesterol (≥69.85 mg/dl) was observed (p-value <0.001). 97.1% of the patients with tubercular pleural effusion had pleural fluid cholesterol levels ≥69.85 mg/dl, contrarily, 42.9% of patients with non-TPE had pleural fluid cholesterol levels below 69.85 mg/dl (Table 4).
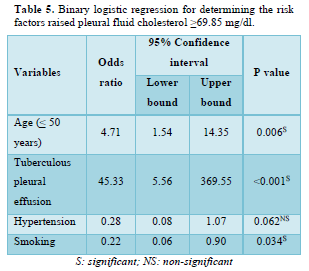
Binary Logistic Regression for Determining the Risk Factors for Raised Pleural Fluid Cholesterol
Table 5 demonstrates the risk factors for raised pleural fluid cholesterol. The strong predictor was TPE, reporting an odds ratio of 45.33 (95% CI: 5.56-369.55). Age (≤50 years) significantly increased the odds by 4.71 (95% CI: 1.54-14.35) of raised pleural fluid cholesterol ≥69.85 mg/dl times. On the contrary, smoking was found to be a protective factor that raised pleural fluid cholesterol.
DISCUSSION
Exudative pleural effusion is most frequently encountered in clinical practice and frequently causes challenging diagnostic issues. For TPE, there are drawbacks to diagnostic methods, such as a dearth of positive pleural fluid staining and cultures and a lengthy identification process. The current investigation aimed to evaluate the possible diagnostic utility of pleural fluid cholesterol in the diagnosis of TPE.
In the present study, the mean age of the TPE and non-TPE groups were 35.54±14.13 and 57.17±17.99 years respectively. In the TPE group, it varied from 18 to 70 years, while in the non-TPE group, it was 18 to 90 years. The mean age of TPE is rising and both middle-aged and old people are typically affected by this disease. A similar age group is observed by Dave [18] and Ungerer [19].
Our study was male predominant. This gender disparity can be attributed to a variety of variables, including biological variations, how diseases manifest, and access to healthcare. Additionally, men have more chance of TB exposure than female. According to the statistics, men are more likely than women to suffer from lung cancer and TB. In the last few decades, the prevalence of lung cancer has increased in females [20]. Smoking is the primary cause of lung cancer and is linked to tuberculosis. Men tend to smoke more often than women. Therefore, smoking has a greater impact on the burden of TB disease in men.
ADA is the most often utilized biomarker for the diagnosis of TPE. However various factors influence the TPE diagnosis and pleural fluid ADA level. In a 2013 study, Tay and Tee et al. discovered that absolute lymphocyte count, age, serum protein, and LDH were all independent predictors of pleural fluid ADA [21]. Age-related declines in ADA level were also demonstrated [15,22,23]. Another study by Kashiwabara [24], in 2012 demonstrated that ADA has a positive correlation with LDH, although there was no discernible correlation between age and serum protein [24].
Pleural fluid cholesterol is now a recognized marker to distinguish between exudative and transudative pleural effusion. The definite cause for increasing cholesterol in pleural exudates is unidentified. There have been two proposed explanations. Firstly, cholesterol is produced by pleural cells for their metabolic requirements, secondly due to the degradation of leucocytes and macrophages, which contain significant amounts of cholesterol, the quantity of cholesterol in the pleural cavity rises [16]. The pattern of TB infection is different from other pathogens because of the presence of distinctive virulence factors. They invade the cell of the host and survive in the phagosomes, which are deficient in nutrition. MTB has an exclusive capacity to utilize cholesterol that frequently appears in mammalian cell membranes, and facilitates the organism's survival inside of macrophages and leucocytes [25]. TB infects the highest area of oxygen concentration as the cholesterol metabolism pathway necessitates a large amount of oxygenase [26].
For MTB, cholesterol transfer is crucial for intracellular growth, hence they are primarily found in a cholesterol-rich region of the macrophages. M. tuberculosis uses the breakdown of cholesterol to turn carbon into energy and impacts the composition and quantity of bacteria essential for virulence [25]. During cholesterol metabolism, different metabolites that could be lethal are produced. The first product of the cholesterol metabolism pathway is cholestenone, which is very lethal, and collection of this toxic cholestenone kills the cell and causes the release of this mobilized cholesterol from membranes into the pleural space. This can explain the increased cholesterol and ADA in pleural fluid in response to inflammation because both ADA and cholesterol are released from cells mainly lymphocytes and macrophages. Cholesterol is the major component of about 30% of lipids of the plasma membrane and is necessary to maintain its fluidity. Cholesterol is required to maintain the secretory process of phagocytic cells like cell motility, exocytosis, and endocytosis [27]. Furthermore, cholesterol catabolism by MTB in macrophages has an essential effect on the host by decreasing the local concentration of membrane cholesterol.
Their phagocytic function might be responsible for low cholesterol levels in the plasma membrane and help MTB to flourish in a conducive environment. The metabolism of lipids in MTB is regulated by 250 genes. These findings imply that M. tuberculosis utilizes cholesterol, which raises the level of cholesterol in TPE.
In our study, ROC analysis has been used extensively to compare pleural fluid ADA with pleural fluid cholesterol. Research on pleural fluid cholesterol evaluation for the diagnosis of TPE has not been widely available. Goyal [28] reported that pleural fluid cholesterol might be useful as a simple marker of TPE. The current study's ROC curve demonstrated that pleural fluid ADA had a sensitivity of 94.2% and a specificity of 62.8%, whereas pleural fluid cholesterol had a sensitivity of 97.1% and a specificity of 57.1%. The statistically substantial similarity between the AUC of pleural fluid cholesterol and ADA raises the possibility that pleural fluid cholesterol may be a novel diagnostic marker for TPE.
LIMITATIONS OF THE STUDY
It was undisputable that this study had a small sample size and was conducted at a single center. The subsequent analysis in different non-TPE such as malignant, parapneumonic, and empyema thoracic groups was not done. As a result, the interpretation of this study’s results is limited. We only explore the diagnostic value of pleural fluid cholesterol. Future multicenter studies with larger sample sizes are required to validate the findings of our study.
CONCLUSION
In summary, pleural fluid cholesterol levels were markedly higher in TPE than in non-TPE. It has significant potential to diagnose TPE and differentiate it from non-TPE. The results of this study support that, pleural fluid cholesterol might be useful as a novel marker for the diagnosis of TPE. Therefore, pleural fluid cholesterol must be included in the diagnostic algorithm of exudative pleural effusion.
- Chakaya J, Khan M, Ntoumi F, Aklillu E, Fatima R, et al. (2021) Global Tuberculosis Report 2020 - Reflections on the Global TB burden, treatment and prevention efforts. Int J Infect Dis 113Suppl (Suppl 1): S7-S12.
- Patel A, Choudhury S (2013) Pleural tuberculosis presented as multiple pleural masses: An atypical presentation. Lung India 30(1): 54-56.
- Vorster MJ, Allwood BW, Diacon AH, Koegelenberg CFN (2015) Tuberculous pleural effusions: advances and controversies. J Thorac Dis 7(6): 981-991.
- Light RW (2010) Update on tuberculous pleural effusion. Respirology 15(3): 451-458.
- Yurt S, Kirkil G (2018) Diagnostic Utility of Adenosine deaminase 1-2, and Interferon-? In the Diagnosis of Pleural Tuberculosis. J Infect Epidemiol 2(1): 1-4.
- Porcel JM (2018) Biomarkers in the diagnosis of pleural diseases: A 2018 update. Ther Adv Respir Dis 12: 1753466618808660.
- Trajman A, Pai M, Dheda K, van Zyl Smit R, Zwerling AA, et al. (2008) Novel tests for diagnosing tuberculous pleural effusion: What works and what does not? Eur Respir J 31(5): 1098-1106.
- Kelam MA, Ganie FA, Shah BA, Ganie SA, Wani ML, et al. (2013) The diagnostic efficacy of adenosine deaminase in tubercular effusion. Oman Med J 28(6): 417-421.
- Chen ML, Yu WC, Lam CW, Au KM, Kong FY, et al. (2004) Diagnostic value of pleural fluid adenosine deaminase activity in tuberculous pleurisy. Clin Chim Acta 341(1-2): 101-107.
- Pai M, Flores LL, Hubbard A, Riley LW, Colford JM (2004) Nucleic acid amplification tests in the diagnosis of tuberculous pleuritis: A systematic review and meta-analysis. BMC Infect Dis 4(1): 1-14.
- Dheda K, van Zyl-Smit RN, Sechi LA, Badri M, Meldau R, et al. (2009) Utility of quantitative T-cell responses versus unstimulated interferon-{gamma} for the diagnosis of pleural tuberculosis. Eur Respir J 34(5): 1118-1126.
- Morimoto T, Takanashi S, Hasegawa Y, Fujimoto K, Okudera K, et al. (2006) Level of antibodies against mycobacterial glycolipid in the effusion for diagnosis of tuberculous pleural effusion. Respir Med 100(10): 1775-1780.
- Aloona SP, Sharma R, Singh B, Neki NS (2017) Diagnostic value of pleural fluid cholesterol versus pleural fluid protein/total serum protein ratio to differentiate transudative pleural effusion from exudative pleural effusion. Int J Curr Res Biol Med 2(4): 1-8.
- Hamm H, Brohan U, Bohmer R, Missmahl HP (1987) Cholesterol in pleural effusions. A diagnostic aid. Chest 92(2): 296-302.
- Valdés L, Alvarez D, San José E, Juanatey JR, Pose A, et al. (1995) Value of adenosine deaminase in the diagnosis of tuberculous pleural effusions in young patients in a region of high prevalence of tuberculosis. Thorax 50(6): 600-603.
- Hamal AB, Yogi KN, Bam N, Das SK, Karn R (2013) Pleural fluid cholesterol in differentiating exudative and transudative pleural effusion. Pulm Med 2013: 135036.
- IBM Corp (2015) IBM SPSS Statistics for Windows, Version 23.0.
- Dave V, Patel SJ, Shah A (2018) Pleural Fluid Adenosine Deaminase for Diagnosis of Tuberculous Pleural Effusion. Int J Res Med 7(2): 41-49.
- Ungerer JP, Oosthuizen HM, Retief JH, Bissbort SH (1994) Significance of adenosine deaminase activity and its isoenzymes in tuberculous effusions. Chest 106(1): 33-37.
- Antonangelo L, Vargas FS, Seiscento M, Bombarda S, Teixera L, et al. (2007) Clinical and laboratory parameters in the differential diagnosis of pleural effusion secondary to tuberculosis or cancer. Clinics (Sao Paulo) 62(5): 585-590.
- Tay TR, Tee A (2013) Factors affecting pleural fluid adenosine deaminase level and the implication on the diagnosis of tuberculous pleural effusion: a retrospective cohort study. BMC Infect Dis 13: 546.
- Yeon KM, Kim CJ, Kim JS, Kim CH (2012) Influence of Age on The Adenosine Deaminase Activity in Patients with Exudative Pleural Effusion. Tuberc Respir Dis (Seoul) 53(5): 530-541.
- Merino JM, Carpintero I, Alvarez T, Rodrigo J, Sánchez J, et al. (1999) Tuberculous pleural effusion in children. Chest 115(1): 26-30.
- Kashiwabara K, Okamoto T, Yamane H (2012) When pleural potassium exceeds 5.0 mEq/L, high pleural adenosine deaminase levels do not necessarily indicate tuberculous pleuritis. Respirology 17(1): 92-98.
- Pandey AK, Sassetti CM (2008) Mycobacterial persistence requires the utilization of host cholesterol. Proc Natl Acad Sci U S A 105(11): 4376-4380.
- Van der Geize R, Yam K, Heuser T, Wilbrink MH, Hara H, et al. (2007) A gene cluster encoding cholesterol catabolism in a soil actinomycete provides insight into Mycobacterium tuberculosis survival in macrophages. Proc Natl Acad Sci U S A 104(6): 1947–1952.
- Ouellet H, Johnston JB, de Montellano PRO (2011) Cholesterol catabolism as a therapeutic target in Mycobacterium tuberculosis. Trends Microbiol 19(11): 530-539.
- Goyal V, Agarwal Y, Singh BS, Abhishek S (2017) Efficacy of Estimating Pleural Fluid Cholesterol in Diagnosing Tubercular Pleural Effusion. Sch J Appl Med Sci 5(10): 4105-4113.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Pathology and Toxicology Research
- Journal of Allergy Research (ISSN:2642-326X)
- Journal of Ageing and Restorative Medicine (ISSN:2637-7403)
- Journal of Otolaryngology and Neurotology Research(ISSN:2641-6956)
- Advance Research on Endocrinology and Metabolism (ISSN: 2689-8209)
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- International Journal of Radiography Imaging & Radiation Therapy (ISSN:2642-0392)